In biotech and vaccine facilities the liquid effluents need decontamination before it can be treated in normal wastewater treatment.
Pharmtec’s patented technology ensures a high resistance to clogging while offering superior, validatable, performance.
We are the only company in the market specialising in high purity applications at the same time as biosafety applications. We base our know-how on more than 50 years of experience of sterilisation in aseptic production, and apply the same stringent demands on biological effluent decontamination.
Made for sterile applications
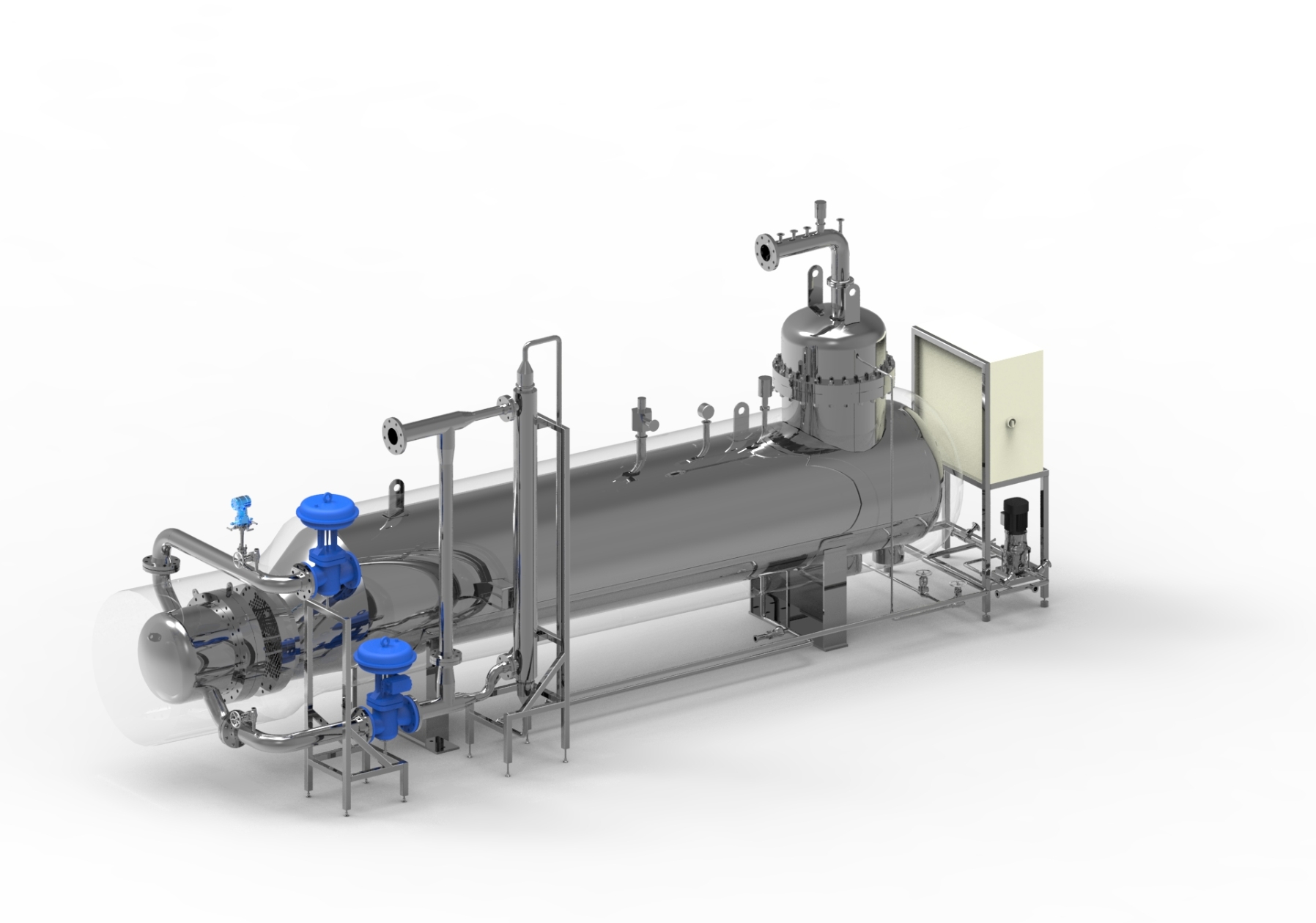
The units are designed as if they were made for sterile applications, with the same stringent respect for the barriers between contaminated and decontaminated effluent as in sterilisation. The decontamination module returns to a safe state after every decontamination cycle.



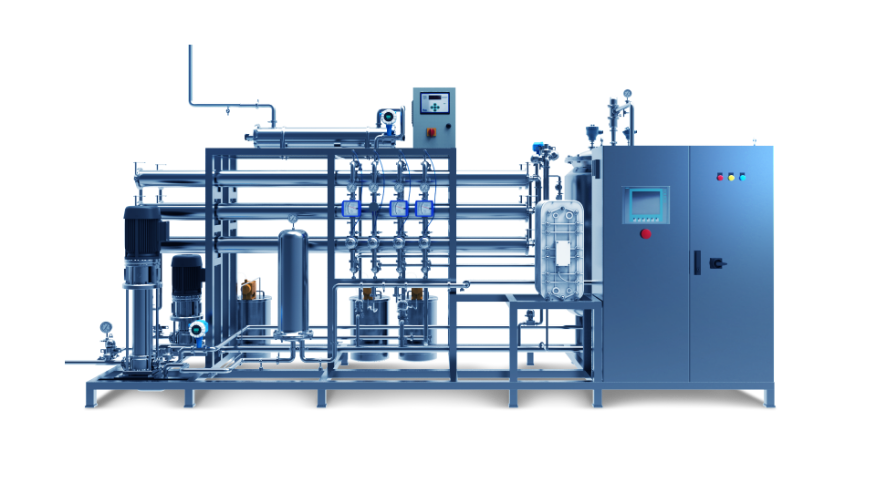

Robust control system
The control system is made with robust batch validation in mind, including all necessary data for correct recording of the decontamination cycles. Input/output testing, manual modes, parameter setting, Access control (with optional Active Directory) and optional 21 CFR 11 compliance, and audit trail.


Mats Mickos
Directuer Général, Pharmtec SA, France
“Nous travaillerons avec votre équipe pour choisir les meilleurs produits et spécifications répondant à vos besoins. Nous pouvons prendre en charge tout, de votre premier appel téléphonique à l’installation.”
Call: + 33 6 77 51 96 26
Email: [email protected]
Micro-Batch SC-200

The revolutionary Micro-Batch series can decontaminate from 1 000 to 10 000 liters per day. The unit has no heat exchanger and no pump, takes a small footprint, and can be adapted to BSL2-3-4 applications. Extremely fast start-up with less than 5 minutes from start to first treatment batch.
| Micro-batch SC-200 | Treatment module |
|---|---|
| Treatment Capacity | Up to 5,000l/day |
| Depth | 1400mm |
| Width | 1,100mm |
| Height | 1500mm |
| Plant Steam | 70 kg/h @ 6 bar g |
| Electricity | 1,5 kW |

