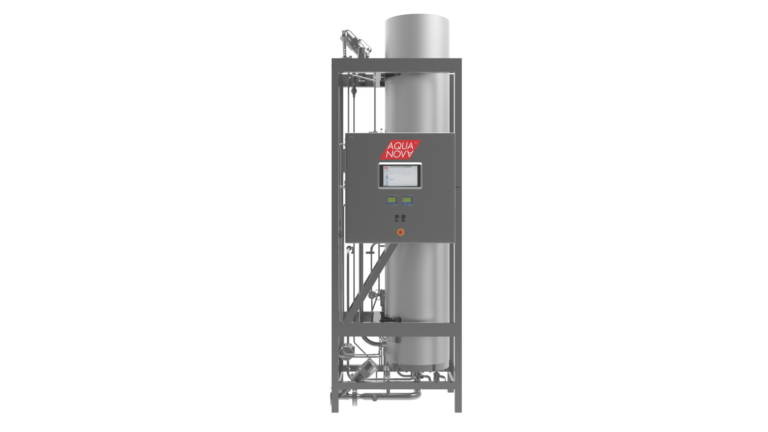
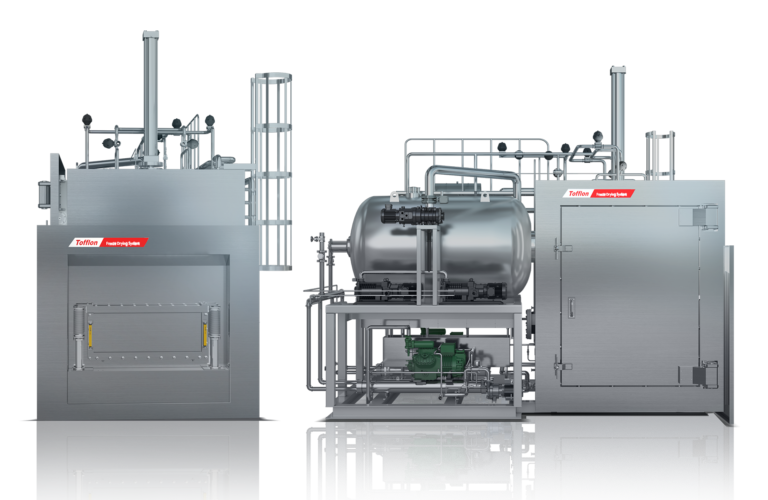
When purchasing a dry heat sterilizer/depyrogenation oven (DHS), it is necessary to clarify the level of performance within the critical criteria for dry heat sterilization and depyrogenation and to clarify the impact on surroundings.
This paper will focus on the following items:
- Environmental classification
- Temperature
- Impact on surroundings